
However, most women experience retrograde menstruation, but only appropriately 10% of reproductive-aged women develop EMs, suggesting that additional mechanisms are involved.
In addition to the theories of coelomic metaplasia, embryonic cell rest, induction and lymphatic and vascular dissemination, the implantation theory, which is based on retrograde menstruation, is the most widely accepted aetiological explanation of EMs it focuses on the pathogenesis of peritoneal and ovarian EMs, postulating that retrograde menstruation delivers sloughed endometrial fragments to the ectopic locations where they implant and grow. To date, the aetiology of EMs is still poorly understood and sometimes controversial despite the progress in EMs research in the last several decades. Therefore, the importance and urgency of understanding EMs pathogenesis is underscored by health concerns everywhere, in order to explore more definitive therapies. However, current treatment strategies, including surgical procedures, medical therapy or a combination of both, fail to prevent high recurrence of EMs and produce serious side effects that negatively affect the fertility and reproductive health of women. Consequently, these ectopic endometrial-like tissues cause not only chronic, intolerable pain (dysmenorrhea, dyspareunia and pelvic pain) but also subfertility or infertility, and these two main clinical symptoms of EMs profoundly affect patient quality of life. EMs is histologically characterized by the presence of endometrial-like tissues (composed of both glandular and stromal tissue) outside the uterus, and this tissue undergoes cyclic regeneration and shedding processes similar to those of the normal endometrium. These cells could be of great help in exploiting promising therapeutic targets and new biomarkers for EMs treatment and prognosis.Įndometriosis (EMs) is an oestrogen-dependent, progesterone resistant, inflammatory, benign gynaecological disorder that is commonly observed in women of reproductive age (5–15% of reproductive-age women and 20–50% of women with chronic pelvic pain and/or infertility).

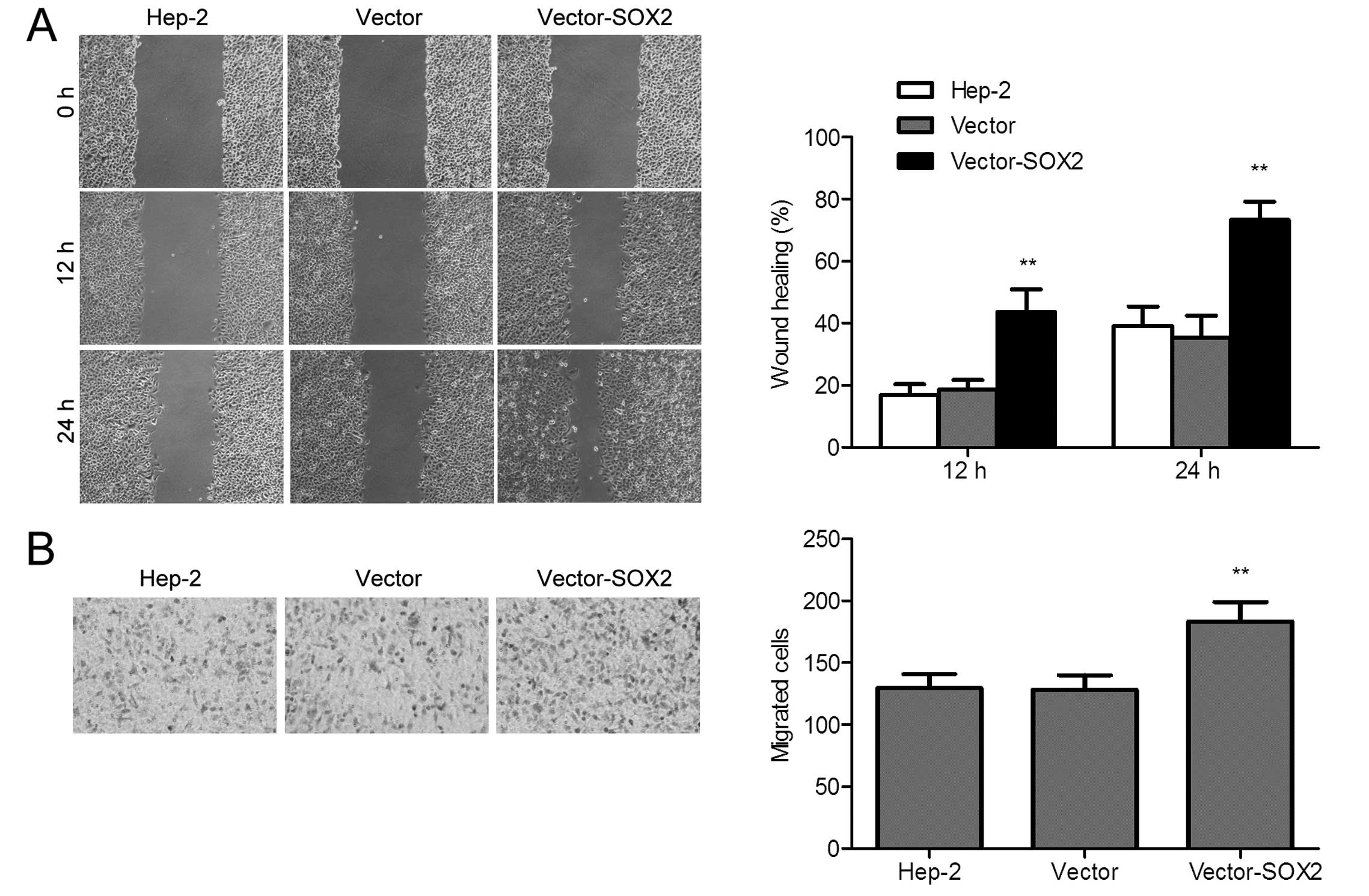
Our results not only improve the understanding of EMs but also contribute to the development of EnSC-EM-EC as a tool for EMs drug discovery. A total of 523 DEGs between EnSC-EM-EC and EnSC-Control were identified and analysed using the KEGG and Gene Ontology databases. Greater amounts of angiogenic factors (especially VEGF and PDGF) were secreted by EnSC-EM-EC than by EnSC-Control however, the distinct profiles of cytokines secreted by EnSC-EM-EC and adhesion molecules expressed by EnSC-EM-EC require further investigation. ResultsĮnSC-EM-EC exhibited unique biological characteristics, including prolonged mitosis, enhanced migratory capacity and enhanced angiogenic potential. Differentially expressed genes (DEGs) between EnSC-EM-EC and EnSC-Control were analysed by RNA-sequence. The expression of 11 angiogenesis-associated biological factors and 11 cytokines secreted by EnSCs and 17 adhesion molecules expressed on EnSCs were determined by protein array assays respectively. Then, the proliferative capacity, migratory capacity and angiogenic potential of EnSCs were evaluated by conventional MTT assay, flow cytometry, wound healing assay, transwell assay, tube formation assay and chick embryo chorioallantoic membrane assay respectively. MethodsĮnSC-EM-EC ( n = 12) and EnSC-Control ( n = 13) were successfully isolated. We further performed preliminary exploration of the potential signalling pathways involved in the above abnormalities. Therefore, we aimed to identify the differences between EnSCs isolated from the ectopic lesions of EMs patients (EnSC-EM-EC) and EnSCs isolated from eutopic endometrium of control group (EnSC-Control).

Currently, accumulating evidence has shed light on the importance of endometrial stem cells (EnSCs) residing in the basal layer of endometrium in the establishment and progression of endometriotic lesions. However, the aetiology of EMs is poorly understood and controversial despite the progress in EMs research in the last several decades. Research into the pathogenesis of endometriosis (EMs) would substantially promote its effective treatment and early diagnosis.


 0 kommentar(er)
0 kommentar(er)
